Now, you might be thinking, “How is that even possible?” Well, that depends on a whole lot of things. Most of the time, temperature and pressure are major factors. In other cases, strange things happen when water is combined with some other substance.
10 Ice-VII
Ice is cold. But not ice-VII (aka hot ice), which is actually hot. Scientists call the regular ice we have here on Earth “ice Ih.” The little “h” there means “hexagon,” since the oxygen atoms line in a hexagon shape when water freezes under normal pressure. However, ice Ih becomes ice-II when more pressure is introduced. Ice-II becomes ice-III when yet more pressure is applied, and it goes on and on until it reaches (or even passes) ice-VII, where the oxygen atoms are arranged in a cubic shape. Ice-VII is hot because it is only formed at a high temperature and pressure. On Earth, it could theoretically only exist deep in the mantle, where the pressure is high enough to compress regular water into ice-VII. However, it will not form in the mantle because the high temperature will turn water into vapor before the pressure can turn it into ice. Scientists have been able to create ice-VII in the lab. They have also discovered it in diamonds formed deep inside the Earth’s mantle. The ice was formed from water droplets got trapped in the diamonds at the time they were formed in the mantle.[1]
9 Dry Water
Dry water is what we get when we mix natural water with silica (with the aid of a machine). It appears and behaves like a solid even though it is 95-percent water. It consists of powdery sugar-like grains, which are actually regular water droplets covered with silica. The silica prevents the water from mixing and becoming liquid. Dry water was first developed in 1968 and was used in cosmetics at the time. Everyone soon forgot about it until researchers from the University of Hull, UK, reinvented it again in 2006. Scientists think dry water could be used to absorb carbon dioxide from the atmosphere. This could work, considering that dry water absorbs three times more carbon dioxide than regular water alone. Scientists are also considering using it for the storage and transportation of harmful chemicals.[2]
8 Supercritical Water
A substance reaches a supercritical state when its temperature and pressure become so high that there is no difference between its liquid and gaseous states. In water, that is after gas. So water goes: solid, liquid, gas, and supercritical—in that order. Water at this point exists as a weird vapor that is not actually a gas. Water reaches its supercritical state at 373 degrees Celsius (703 °F) and 220 bars in pressure. It cannot be compressed back to a liquid in that state.[3] Supercritical water (as with other supercritical fluids) can effuse through a solid like a gas but can still dissolve other substances like a liquid.
7 Plasma Water
Gliese 1214 b is one weird planet. It is six times larger than the Earth and filled with water—including plasma water. That is, water existing in a plasma state.[4] Matter in a plasma state is a bit similar to a gas. It has a low density and no definite shape or volume—just like gas. However, unlike gas, the matter’s atoms have had their electrons stripped away, and the positively charged nuclei move around freely. This is why some scientists consider plasma the electrically charged version of gas. Back to Gliese 1214 b. The planet is so close to its star that a year is just 38 hours long. Earth is 70 times farther from our Sun by comparison. Daytime temperature on Gliese 1214 b could reach 282 degrees Celsius (540 °F), which is way too hot for almost anything to survive. The proximity of Gliese 1214 b to its star is the reason water may exist as plasma over there. The extremely high temperature from the star and the high pressure of the planet causes water to heat and compress so much that it becomes plasma. Plasma water is considered one of the supercritical forms of water we mentioned earlier.
6 Triple Point Of Water
The triple point of a substance is defined as the conditions where a substance’s solid, liquid, and gaseous states can all exist in thermodynamic equilibrium. This can only happen when that substance reaches a specific temperature and pressure. For water, that temperature and pressure is 273.16 degrees Kelvin (0.01 °C, 32.02 °F) and 611.66 pascals (6.1166 mbar, 0.0060366 atm), respectively.[5] The triple point of water is used to determine the temperature in Kelvin, calibrate thermometers, and establish the triple point of other substances. Water at its triple point can be turned into solid, liquid, or gas by just adjusting its pressure and temperature accordingly.
5 Superionic Ice
Superionic ice, or ice-XVIII, is another form of ice formed by a massive increase in temperature and pressure. It is hot, black, dense, and behaves like metal. A solid cube of ice-XVIII is four times heavier than an equivalent solid cube of regular ice. Some scientists believe ice-XVIII may be the most common form of water in the universe, existing in “ice giant” planets like Uranus and Neptune. Interestingly, scientists only confirmed ice-XVIII’s existence in 2019, even though it was predicted in 1988. That year, a group of scientists revealed that water would behave like metal if its temperature and pressure got high enough. Ice-XVIII only forms when the temperature is thousands of degrees and the pressure is at millions of atmospheres. Scientists confirmed ice-XVIII in an experiment in which they used powerful lasers to create shock waves that rapidly increased the temperature and pressure of a droplet of water. Scientists observed that the hydrogen and oxygen molecules in the water instantly separated as the water turned into crystalline ice. The oxygen molecules formed frozen, solid structures called cubic lattices, while the hydrogen atoms flowed like a liquid around the hardened oxygen. Some scientists say this so-called “ice” cannot be considered water because the hydrogen and oxygen molecules separated. They say the hydrogen and oxygen molecules need to be together for it to be considered water.[6]
4 Aeroice
Aeroice is the lightest version of ice out there. It was “discovered” in a simulation in 2017 by researchers at Okayama University in Japan during an experiment to understand how water transforms into ice. The research team created the ice when they tried to find out what happened when water freezes in the absence of pressure. The other phases of ice we have mentioned so far were created after extreme pressure was applied to water. This simulation looked at negative pressure. The scientists “created” aeroice by extracting the two atoms of oxygen in silicon dioxide (aka silica), leaving behind just the silicon. Then they replaced the silicon atom with an oxygen atom before introducing two hydrogen atoms to create ice.[7] This finding may have implications for how water behaves in nanotubes, nanopores, or others parts of the cosmos.
3 Amorphous Ice
Amorphous ice is created by rapidly cooling liquid water so that the molecules don’t have time to form a crystal lattice. Lacking normal ice’s ordered crystalline structure, amorphous ice is generally considered a glass, it that it’s a liquid whose movement is just insanely slow. Amorphous ice is not common on Earth but is believed to be the most abundant form of water in the universe.[8] A 2017 study involving computer simulations of amorphous ice implied that glasses may represent a state somewhere between crystalline and liquid. The simulated amorphous ice displayed disordered hyperuniformity, in which there is order across large spatial distances but none across short distances.
2 Burning Ice
Methane hydrates are a kind of ice that can actually burn, as in you can set it alight like a piece of paper. The ice in question contains methane. It naturally forms at certain ocean depths, in permafrost, and even in oil and gas pipelines, where it can cause blockages. That last bit is how it was discovered, back in the 1930s. Burning ice begins as compressed and frozen methane. The frozen methane is soon covered with ice, creating burning ice. Scientists consider the ice a possible source of fuel, considering that it contains lots of methane. A cubic meter of burning ice can release 160 cubic meters of methane. It’s also cleaner than coal. Unfortunately, many countries cannot replace their coal with burning ice because it is difficult to extract underwater. It also becomes unstable when brought to the surface. Scientists say burning ice could also work the other way and exacerbate climate change. This would happen when methane hydrate-containing permafrost melts and releases methane into the atmosphere.[9]
1 Quantum Water
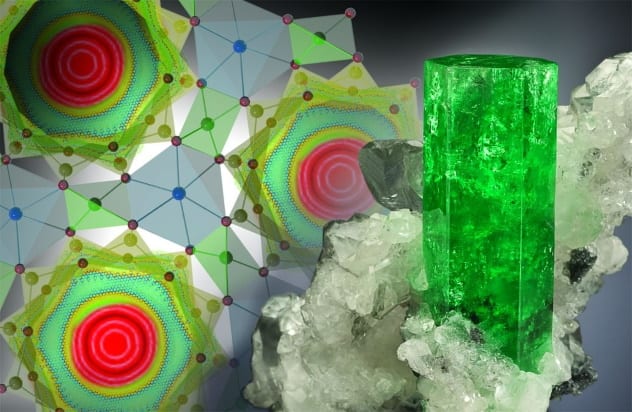
In 2016, scientists at the United States Department of Energy’s Oak Ridge National Laboratory created a new quantum state of water. They made the discovery by “squeezing” water molecules in between hexagonal beryl crystals. The massive compression increased the pressure so much that the atoms of the water molecules became misaligned, at which point the water no longer followed a number of physics laws. The molecules were able to pass through barriers at the atomic level, a behavior that is explained by quantum mechanics and referred to as “tunneling.” This behavior only occurs when a substance is in a quantum state. Scientists believe water often goes into quantum mode to travel through very tight spaces in rocks, soil, and even the walls of living cells.[10]